Introduction
The investigation of separation performance of porous materials is very important for evaluation of such materials for separation
processes. The separation of methane and carbon dioxide was studied on activated carbon as it appears in many applications such as biogas upgrading or natural gas cleaning. For evaluation of the separation performance, breakthrough curves of a ternary mixture (methane, carbon dioxide, and helium) were acquired using the mixSorb L analyzer (3P INSTRUMENTS) as seen in Figure 1 coupled with a ThermoStar MS (mass spectrometer, Pfeiffer). It is shown that the mixSorb L measurements allow for a better understanding of mixed gas adsorption processes as compared to static gas sorption characterization methods. Furthermore, these measurements provide the basis for the calculation of the concentration profiles of a fixed bed adsorber, linking the development of new adsorbents and their use in industrial practice.

Figure 1. Automated instrument mixSorb L for determination of breakthrough curves.
Experimental
For measurement of a breakthrough curve, a gas flow with a well-defined concentration of both adsorptives in a carrier gas passes through a fixed bed adsorption column containing the sample. The concentrations of all gases at the outlet of the fixed bed are detected in a time-resolved manner. A dead volume correction can be applied by measuring the response signal during an experiment with inert material, which will not adsorb the adsorptive gas. Approximately 50 grams of activated carbon A was used; the details of the experiment are presented in Table 1. The end of the breakthrough was defined by a time criteria with a maximum measurement time of 120 minutes and a temperature stability criteria of 0.5 K in 10 minutes.
Table 1 Experimental Conditions for Separation Test.
Experimental Condition
Value
Sample mass (activated carbon)
50.52 g
Carrier gas (helium)
80 Vol. %
Adsorptive 1 (methane)
15 Vol. %
Adsorptive 2 (carbon dioxide)
5 Vol. %
Total gas flow
2500 mL/min (STP)
Temperature
20 °C
Pressure
5 bar
The adsorber column of the mixSorb L was filled with activated carbon. After connection of the adsorber column, a gas flow with 0.3 L/min helium was applied to flush impurities and pre-adsorbed moisture off of the sample before a leak test was performed. The column was heated at 5 K/min up to 120 °C, the temperature was then held at 120 °C for 3 hours, and finally the system was cooled down to the measurement temperature of 20 °C. The MS-signals of the pure carrier gas and the inlet gas mixture were acquired by a bypass measurement. The adsorber column was then pressurized to the desired pressure using the helium carrier gas. Due to compression, the temperature inside the adsorber column increased, so the adsorber column was cooled to the desired measurement temperature. After reaching thermal equilibrium, the analysis was started by flowing the desired gas mixture while acquiring the MS-signal of the adsorber effluent. After the measurement, the column was flushed with 0.5 L/min nitrogen for 1 hour to remove helium and carbon dioxide from the adsorber column before weighing to determine the post-analysis sample mass.
Results
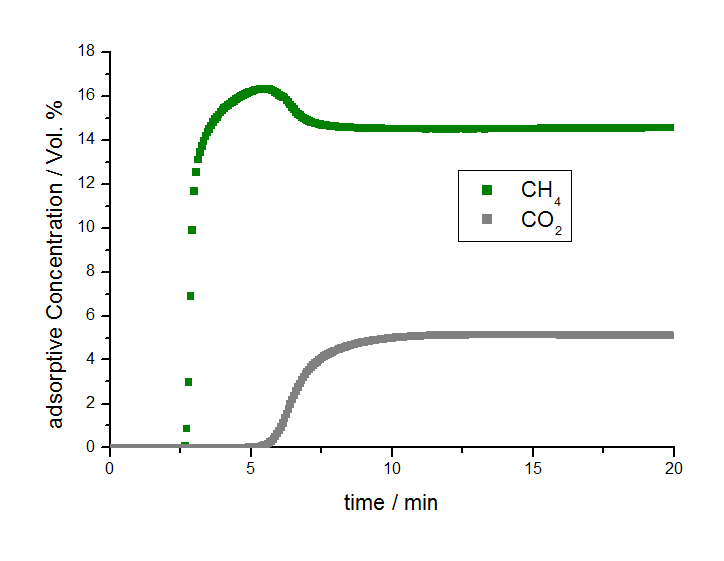
Figure 2 Illustrates the results of the separation experiment for methane and carbon dioxide. Here it can be seen that the breakthrough of methane occurred after approximately 2.5 minutes under the studied conditions while the breakthrough of carbon dioxide was observed after more than 5 minutes. Therefore there is a separation process between methane and carbon dioxide and within a specific time frame, between 2.5 and 5 minutes in this case, pure methane in helium flows out of the adsorber column before carbon dioxide breaks through.
Conclusions
There is an obvious separation effect by the activated carbon A. It has been shown that methane has a substantially shorter breakthrough time compared to that of carbon dioxide, thus the methane gas can be separated in the time interval between the two gas breakthroughs. The mixSorb L coupled with a mass spectrometer offers the ability to study the adsorption behavior of the components of ternary gas mixtures and can be used as an experimental tool to optimize porous materials for specific gas separation applications. Such valuable performance characteristics can only be derived from dynamic experiments.